The Science of Composting

What kinds of microorganisms are necessary for successful “good” soil production?
The composting process is carried out by three classes of microbes
• Psychrophiles - low temperature microbes
• Mesophiles -medium temperature microbes
• Thermophiles - high temperature microbes
• Mesophiles -medium temperature microbes
• Thermophiles - high temperature microbes
* Generally, composting begins at mesophilic temperatures and progresses into the thermophilic range.

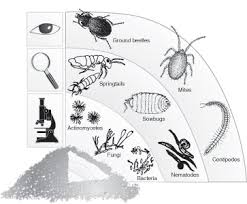
What kinds of microorganisms/invertebrates assist in composting?
Composting microorganisms include Actinomycetes, Streptomyces, Micromonospora, Centipedes, Millipedes, Fungi, Sowbugs, Molds, Spiders and Earthworms.
REMEMBER:
What is a microorganism?
When I use the term microorganism I am recalling the millions, billions, and trillions of creatures making up the largest number of living organisms on the planet! Not only are they “microscopic” in size, meaning very small for our naked eyes to see but they work alone or in colonies which can either affect you negatively or positively.

REMEMBER:
Microorganisms are important to soils because…
- Responsible for cycling of C, N and other nutrients
- Enhance soil structure
- Relocate and decompose organic materials
- Maintain soil quality and health
- Increase soil aeration and penetrability
- Involved in disease transmission and control

Temperature and its effects on Composting
Temperature is directly proportional to the biological activity of these different microbial species within the composting system. As the metabolic rate of the microbes accelerates the temperature within the system increases whereas if the metabolic rate of the microbes decreases, the system temperature decreases.


DID YOU KNOW?
- At a temperature of 130 degrees Fahrenheit for approximately 3-4 days, favors the ruining of weed seeds, fly larvae and plant pathogens.
- At a temperature of 155 degrees Fahrenheit results in the decomposition of organic matter about twice as fast as at 130 degrees Fahrenheit.
- At temperatures above 155 degrees Fahrenheit may result in the death of certain microbe species which simultaneously would decrease the temperature.
Composting microorganisms thrive in moist conditions.

KEEP IN MIND…
- As the microbial population increases, therefore the temperature of the soils increases.

CHECKPOINT…
What are some possible factors that influence temperature?
1. Moisture content
2. Oxygen availability
3. Microbial activity
When you begin to compost, you are going to see a pile of organic waste pile on top of each other. When the pile increases, so does the temperature which in this situation is ideal for “good” soil production. There is a climax in which the temperature peaks and begins to decrease. At this point, the pile desires the addition of oxygen into the compost. During the composting process oxygen is used up quickly by the microbes as they metabolize the organic matter. Aerating the compost by turning should ensure an adequate supply of oxygen to the microbes. By turning the compost sparingly, like a mother turning soup in a pot, the oxygen will aid in the temperature of the pile peaking again, thus initiating the cycle of organic decomposition once again. This “turning” process should be continued until the compost pile fails to re-heat. This result indicates desired biologically stability of your compost materials.

CARBON NITROGEN
RATIO

All organic matter is made up of substantial amounts of carbon (C) combined with lesser amounts of nitrogen (N). The balance of these two elements in an organism is called the carbon-to-nitrogen ratio (C:N ratio). For “good” soil microorganisms require the correct proportion of carbon for energy and nitrogen for protein production. Scientists have determined that the fastest way to produce fertile, aromatic compost is to maintain a C:N ratio somewhere around 25 to 30 parts carbon to 1 part nitrogen, or 25-30:1. If the C:N ratio is too high (excess carbon), decomposition slows down. If the C:N ratio is too low (excess nitrogen) you will end up with a stinky pile.
- Below are the average C:N ratios for some common organic materials found in the compost bin.
Following are
some sample C:N ratios
of organic matter:
Sandy loam (fine)
|
7:1
|
Humus
|
10:1
|
Food scraps
|
15:1
|
Alfalfa hay
|
18:1
|
Grass clippings
|
19:1
|
Rotted manure
|
20:1
|
Sandy loam (coarse)
|
25:1
|
Vegetable trimmings
|
25:1
|
Oak leaves
|
26:1
|
Leaves, varies from
|
35:1 to 85:1
|
Peat moss
|
58:1
|
Corn stalks
|
60:1
|
Straw
|
80:1
|
Pine needles
|
60:1 to 110:1
|
Farm manure
|
90:1
|
Alder sawdust
|
134:1
|
Sawdust weathered 3 years
|
142:1
|
Newspaper
|
170:1
|
Douglas fir bark
|
491:1
|
Sawdust weathered 2 months
|
625:1
|


DID YOU KNOW?
Four special types of garbage bins are needed in order to compost indoors like
classrooms and homes:
- Garbage Can Bioreactors- can process enough organic matter to fill a 20-gallon can, producing finished compost within two to three months.
- Soda Bottle Bioreactors- are designed primarily as tools of research rather than waste management. They are small and inexpensive enough to enable students to design and carry out individualized research projects, comparing variables such as reactor design, moisture content, and nutrient ratios of mixtures to be composted.
- High-Tech Bioreactors- are also designed for research purposes. They are similar to the less complicated 2-can or soda bottle bioreactors, but they use more sophisticated technology for measuring temperature and regulating air flow.
- Worm Bins- are another form of indoor composting. Here the decomposition is accomplished by redworms as well as microorganisms, and the temperatures do not get as high as in the bioreactors above. Worm bins are popular in elementary school classrooms, where the students use them for activities ranging from scientific measurement to story writing.

REMEMBER:
For effective composting to occur indoors in certain bins
there needs to be proper conditions for aerobic, heat-producing composting to
occur. Composting can also be carried out right in the classroom, in containers
ranging in size from soda bottles to garbage cans.

Nitrogen Cycle

- Nitrogen is the most common gas found in the earth's atmosphere. It is necessary for plant growth.Free nitrogen is found in the atmosphere, animal wastes, and dead and decaying organisms. However, only a few organisms can use it just as it is. These organisms "fix" the nitrogen for all other organisms to use. They are called nitrogen fixing bacteria.
- In the nitrogen fixation part of the cycle, nitrogen-fixing bacteria found in the soils and in the roots of certain plants, change (or convert) free nitrogen into substances that other organisms can use. When the fixing process is finished, free nitrogen is converted into nitrates, nitrites, and ammonia. These substances can be used by plants. As the plants become food, the nitrogen can be used by animals.
- Just as there are nitrogen-fixing bacteria, some bacteria have the job of denitrifying the soil to keep the nitrogen in balance. These bacteria take the nitrogen compounds and return them to nitrogen gas that is released back into the atmosphere.

DID YOU KNOW?
Nitrogen can be
conserved!!!
There are three phases in the relation of nitrogen supply and conservation to available carbon in biological decomposition:
- When more nitrogen is available than necessary for organisms to use carbon, large quantities of ammonia and volatile forms of nitrogen are given off and lost;
- When the requisite amount of nitrogen to carbon for bacterial utilization is present, decomposition proceeds without appreciable loss of nitrogen;
- When nitrogen is low in relation to carbon, some of the organisms will die and their nitrogen will be recycled. Small additional amounts of nitrogen may be picked up by nitrogen fixation when conditions are satisfactory
Form of nitrogen
|
Source
|
Availablity to plants
|
Remarks
|
Organic nitrogen
(Proteins, amino acids)
|
• Animal manure
• Compost
• Plant residues
• Blood meal
• Many others
|
Not available until broken down--weeks to years
|
Immobile in soil. Slowly converted to NH4+ in
soil.
|
Urea
|
• Commercial fertilizer
• Fresh manure
|
Available fairly quickly as ammonium.*
|
Rapidly converted toNH4+ in soil.
|
Ammonium (NH4+)
|
• Chemical fertilizers such as ammonium nitrate & ammonium
sulfate
• Fresh manure
• Breakdown of organic matter in soil
|
Used directly by some plants; more so under acidic conditions.*
|
Can adsorb to clay or organic matter, reducing leaching.
Converted toNO3- by soil organisms.
|
Nitrate (NO3-)
|
• Chemical fertilizers such as ammonium nitrate & potassium
nitrate
|
Used directly by most plants.*
|
Highly mobile in water. Easily leached to ground-water.
|
Nitrogen gas (N2)
|
• About 80% of air within soil spaces
|
Only available to plants with nitrogen-fixing bacteria, such as
legumes.
|
Organic nitrogen andNH4+ are added to
soil from legumes.
|

What are the
applications of composting?
Compost can be used in a variety of
applications. High quality compost can be used in agriculture, horticulture,
landscaping and home gardening. Medium quality compost can be used in
applications such as erosion control and roadside landscaping. Low quality
compost can be used as a landfill cover or in land reclamation projects.

 CHECKPOINT:
CHECKPOINT:
Remember, you can start learning how to
compost in schools!
Printing off charts (such as the chart
below) can be a great way of remembering how to dispose certain kinds of
materials that we may use daily at home and even schools.
Yard Debris
|
Food Scraps
|
Food Soiled Paper
|
Grass, leaves, weeds
|
Meat, poultry, fish, beans
|
Greasy pizza delivery boxes
|
Pine needles, pine cones
|
Dairy products
|
Coffee filters
|
Thatch,vines
|
Fruit, vegetables
|
Paper towels, napkins
|
Plant trimmings
|
Breads, grains, pastas
|
Paper egg cartons
|
Small amounts of sod - no rocks
|
Eggshells, nutshells
|
Uncoated paper plates, paper cups
|
Branches less than 3" in diameter
|
Coffee grounds, tea bags
|
Paper berry cartons
|
Paper grocery bags with food scraps
|



Very nicely explained about composting, I am sure Kids must inspire from this blog.And they must use composting after reading this blog.
ReplyDelete